how many valence electrons does cesium have|Cesium Valence Electrons (And How to Find them?) : Tuguegarao There are two ways to find the number of valence electrons in Cesium (Cs). The first is to use the Periodic Table to figure out how many electrons Cesium . For most no deposit bonuses, this time frame is 7 days, but it can sometimes be down to just 24 hours for slot spins and similar promotions. How We Find, Review and Rank the Best No Deposit Bonuses. At Zamsino.com, we check and rank the best no deposit bonuses in South Africa from a player’s perspective.
PH0 · Valency of Cesium
PH1 · Valences of the Elements Chemistry Table
PH2 · Valence Electrons Chart for All Elements
PH3 · How to Find the Valence Electrons for Cesium (Cs)
PH4 · How Many Valence Electrons In Cesium?
PH5 · Determine valence electrons using the periodic table
PH6 · Cesium Valence Electrons (And How to Find them?)
PH7 · Cesium (Cs)
PH8 · Caesium Electron Configuration (Cs) with Orbital Diagram
PH9 · 3.10: Valence Electrons
Atlantic City is more than just its top-notch gambling With its beachfront boardwalk, Michelin-starred dining, first-rate casinos, and cool art scene, Atlantic City offers a great weekend getaway for all types of travelers.
how many valence electrons does cesium have*******Mar 23, 2023 Cesium in Periodic table. Cesium element (also spelled as caesium) is in group 1 .how many valence electrons does cesium have Cesium Valence Electrons (And How to Find them?) Learn how to find the valence electrons and valency of cesium, a highly reactive alkali metal with atomic number 55. The valence electrons of cesium are one . There are two ways to find the number of valence electrons in Cesium (Cs). The first is to use the Periodic Table to figure out how many electrons Cesium .
Find the valence of cesium and other elements in this table based on the bond .
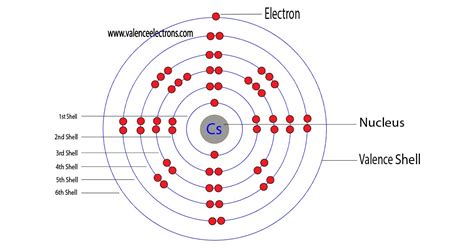
Cesium has 1 valence electron because there is 1 electron present in the outermost shell of the Cesium (Cs) atom. Now let’s see how you can easily find the .In the case of cesium, it is located in period 6, which means that its valence electron is found in the sixth energy level. Since the alkali metals’ valence electron configuration is .CHE 183: Principles of Chemistry I. 3: Electronic Structure and the Periodic Law. 3.10: Valence Electrons. Expand/collapse global location. 3.10: Valence Electrons. Page . So, Caesium electron configuration is basically 1s22s22p63s23p64s23d104p65s24d105p66s1 in its descriptive long written form. The .
Method 1: From the Periodic Table. To find out the valence electrons of Cesium, you have to see the position of cesium in the periodic table. More specifically, you have to see the group wise position of Cesium element in the periodic table. From the above image, you can see that Cesium (Cs) is present in the group 1 of periodic table.Members of a group typically have similar properties and electron configurations in their outer shell. . (GPS) satellites. They give the standard measure of time: the electron resonance frequency of the caesium atom is 9,192,631,770 cycles per second. Some caesium clocks are accurate to 1 second in 15 million years. Biological role. Caesium .Cesium is silvery-gold, soft, ductile alkali metal. It is liquid in a warm room, melting at 28.4 o C (83.1 o F). Cesium is one of the few metals that is liquid near room temperature. The others are gallium, francium and mercury. .The element Cesium was discovered by R. Bunsen and R. Kirchhoff in year 1860 in Germany. Cesium was first isolated by C. Setterberg in 1882. How many valence electrons does a Cesium atom have? Cesium has 1 valence electrons. Cesium has 55 electrons out of which 1 valence electrons are present in the 6s1 outer orbitals of atom. English. ₹ 48,400 (9% Off) ₹ 44,000 per year. Select and buy. The number of valence electrons of caesium is:A.1B.2C.6D.3. Ans: Hint: Caesium belongs to the s-block elements whose last electron enters in \\ [ns\\]-orbital. The atomic number of caesium is 55 and its penultimate shell contains 8 electrons.Complete . A cesium atom has 1 valence electron. It is an alkali metal, and all alkali metals have 1 valence electron. The electron configuration for cesium is (Rn)7s1. The single electron in the 7s sublevel . This table of element valences includes the maximum valence and most common valence values. . of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences. Remember that an element's electron cloud will become more stable by .
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .Comprehensive information for the element Cesium - Cs is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Valence Electrons: 6s 1 Electron Dot Model. Chemical Properties of Cesium. Electrochemical Equivalent: 4.9587g/amp-hr . How many valence electrons does Caesium have? Caesium belongs to the family of Alkali group elements. It has physical appearance as the golden and silvery soft element. Caseium gets the liquid state at the standard room temperature just like some other elements of its category. It has chemical properties similar to Rubidium and the .
Give the electron configuration for sodium. How many valence electrons does it have? 1. How many protons are in an ion with 36 electrons and a -1 charge? 2. How many total electrons are in a Cu^{2+} ion? Based on the octet rule, magnesium most likely forms what ion? Write the electron configuration of Mg+2 ion. 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Cesium have? In the case of Cesium the valence electrons is 1.
how many valence electrons does cesium have Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons. Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .A neutron has approximately the same mass as (1) an alpha particle, (2) a beta particle, (3) an electron, (4) a proton. 1 / 4. Find step-by-step Chemistry solutions and your answer to the following textbook question: How many valence electrons does cesium (Cs) have?.

The electronic configuration of cesium is 6s 1. The electrons in per shell of an atom of cesium are 2, 8, 18, 18, 8 and 1. Cesium atom loses one electron to combine with other atoms and to become stable. When a cesium atom loses one charge, its outer most shell is filled and it becomes a positive ion. Thank Writer.
bokep indo xpanas kimaya agata mendesah Ngentot Memek Tembem Crot di Dalam Viral, BokepOnlineStreaming Gudang Bokep indo,Koleksi vidio bokep Lokal Twitter, bokep indonesia, download bokeb selebgram, perselingkuhan ngentot istri teman, download videobokepgratis bokepcom pembantu, vpn , hijablink, bokep abg, terong tidur sleep, .
how many valence electrons does cesium have|Cesium Valence Electrons (And How to Find them?)